MIDRIB
Building a Digitised Medical Image Library
Copyright 1998 of St George’s Hospital Medical School on behalf of JISC and the MIDRIB Project.
With acknowledgements to the University of Bristol and the Wellcome Centre for Medical Education. Also to Professor Len Doyal of Barts and the Royal London for his input into the Code of Responsible Practice for the Inclusion of Medical Images.
Contents Page No. Introduction 4
a Core images for the curriculum 6
b Pre-existing collections 6
c Commissioning collections 6
d Negotiation with potential donors 7
i What the project provides for donors 7
ii What the project requires of donors 7
- Commitment to the process
- Copyright status
- Ethical clearances
- Description of image content
iii Contract issues 8
- Copyright
- Ethics
- The two-stage contract
a MIDRIB receives Interim Donor License (IDL) and packet of images 15
i Slides examined and counted
ii Collection and IDL stored to be processed b Digitisation 15
i Digitiser selects collection
ii System generates:
- Batch worksheet
- Slide labels iii Labels applied to individual slides
iv Batch digitised
v Store digitised images as batch
vi Transfer digitised batch to temporary location c Convert image format 17
d Digitiser fills and signs batch log sheet 17
e Quality control on batch 17
f Generate Donor License Agreement (DLA) 17
g Generate Image Description forms 17
h Store images in MIDRIB system as locked files 17
i Pack slides (still labelled) for return 17
j Send slides with Donor Licence Agreement and ID forms to donor 18
3 Phase 3 - Cataloguing and Indexing Page No.
a Donor receives originals, DLA with appendices & ID forms with guidelines
b Donor completes forms according to guidelines 19
c Information entered from forms into database — tied to digitised
Image by bar code on form. 19 d Data entry Quality Control 19
e Information used to index the image appropriately in MeSH. 20
f Indexing Quality Control 20 g Search and retrieval issues 20
4 Phase 4 — Images made available
a Donor signs both copies of DLA and each page of appendix B, and
returns one copy to the project 23
b Donor states in DLA that our ethical standards are met and that
patient consent proofs will be sent; or outlines the ethical situation
or each image where the situation is less clear-cut. 23
c All images subject to ethical doubt must be passed via the procedures
laid down by the project’s Medical Ethics Panel before inclusion. 23
d In an unforeseen circumstance, the images will be laid before the
Medical Ethics Panel and an appropriate procedure devised. 24
e CD of images plus catalogue and indexing information returned to donor
f Where licensing and ethical conditions are met, images are made
available through the resource after 30 days. 24 5 Phase 5 — user licensing
a Authorised use 26
b Institutions register one or two authorised individuals who will
register staff and students to use the project 26 b Terms and conditions of use 27
c Levels of access 27
d Termination of access 28 6 Image review
a Logging usage patterns 29
b Deleting unused images after a period 29
c Capturing unsuccessful searches for proactive collection building 29
d Online comment and submission 29 Annexes
Annex A — Interim Donor License (IDL) & covering letter 31
Annex B — Donor License Agreement (DLA) & notes 35
Annex C — Code of Responsible Practice for the Inclusion of Medical Images 40 Annex D — Image description forms 48
Annex E — Patient Consent Forms 49
Annex F — Guidelines for Contributors 53
Annex G — Process Flowcharts, etc 58
MIDRIB: Building a digitised
medical image library
Introduction
This document describes the process developed by MIDRIB for building a digitised library of medical images, from locating appropriate material through to making them available over the Internet.
There are many difficult problems for anyone approaching such a project cold. Ensuring that copyright ownership is correctly identified and obtaining a license for use; that the patient whose image is to be made available has given their informed consent; knowing which images can be used without such consent; how the digitisation process itself is handled; cataloguing and indexing the images for efficient retrieval and storage; all these and more need to be addressed and decisions made on the project’s approach to dealing with them. We hope that the results of our research into all these issues will make the process simpler for UK medical schools embarking on similar projects.
After two years of
detailed consideration, MIDRIB has developed a system incorporating
legally appropriate and economically feasible strategies for handling
these issues. They are outlined in the following pages, following the path
of an image through the system:
There follow additional
sections on image review, resources required for the project and some
technical discussion. Discussion on the issues is included in the text,
and supporting documentation (such as recommended contracts and forms) are
included in the annexes. Each project will have slightly differing aims,
and so care must be taken to ensure that the elements of our processes
adopted still serve the project for which they are employed. This
functional specification should help to clarify these issues.
MIDRIB was funded by
JISC under the Electronic Libraries Programme (eLib) from 1st April 1996
to 30 June 1998, and led by St George’s Hospital Medical School. We
wish to thank the following for their input into the project:
a) Core images for the curriculum
In order to provide a resource containing relevant and useful images, experienced teachers, AV staff and others must work together to produce lists of those images which students must see at some point in their medical education. The same procedure must be carried out for professions allied to medicine (PAMs] and dentistry students.
In addition, unsuccessful searches (those where the resource does not contain an example of what the user requests) are stored, as an additional source of images required by users.
These two sources taken
together enable the practical targeting of donors.
b) Pre-existing collections
Over the past two years, many medical professionals with an existing collection have heard of MIDRIB and made contact with a view to preserving their work and making it available to a wider audience. These collections were nearly always in 35mm slide form, which is convenient because they may easily be digitised using automated equipment (see Phase 2). Unfortunately, as a result of having been built up over a long or longish career in medical practice, they tend to be composed largely of rare or obscure subject matter since this is more interesting to the practitioner or researcher. In addition, the ethical circumstances of their acquisition (and often their actual ownership) is usually uncertain. Such collections, though interesting, will be more appropriate for the resource once the basic core images have been covered.
MIDRIB recommends
that the existing collections of experienced medical teachers be included
first, as these are tried and tested in the educational arena and are
likely to be directly relevant to users. However, the ethical and
copyright considerations outlined in this document still apply.
c) Commissioning collections
The lists of images required can be used to target potential donors working in specific areas. These may be medical professionals or other individuals such as medical photographers. This type of approach guarantees that provenance and thus ownership of the images are clear, and appropriate patient consent can be obtained at the time of image creation. Where necessary, MIDRIB’s ethical code in Annex C can be adopted by the institution’s ethical committee before work starts; this in turn facilitates further commissioning at the same institution.
MIDRIB recommends
that this proactive approach be used wherever possible.
d) Negotiation with potential
donors
A degree of commitment is required from donors at quite an early stage, since inclusion of images in the resource requires considerable work for both the team and the donor. The project’s negotiator must build up this commitment without at any stage minimising the effort required of the donor (sorting and selecting slides, providing copyright and ethical guarantees, additional textual information, and checking) since all stages must be complete before images can be added to the resource. The importance of the donors’ commitment cannot be stated too strongly, since their images are the library and without willing donors no progress can be made.
MIDRIB recommends
that the commitments made by each side are clearly and explicitly stated
early in the negotiation. These are summarised below:
i) What the project provides for donors
The project will digitise the donor’s slides, cleaning and remounting them where necessary, and return them with an identifying label which links them to catalogue/index data in the project resource. On request, the project may provide a CD of the donor’s images with associated catalogue/index data.
Many owners have had it in mind for a long time, often years, to go through their images systematically or to proactively build a coherent, useful and indexed collection. A project of this kind provides the impetus for this action, and supports it by the provision of commissioning lists, cataloguing, indexing and digitising expertise, and (for particularly important collections) may provide an appropriate level of financial support for short periods of additional staffing.
Finally, it appears
that many donors regard inclusion of their images in a resource of this
kind as conveying prestige, and therefore see it as a good thing in its
own right.
ii) What the project requires of donors
This is essential, since donors will have to undertake considerable work to assist in making the images useful in practice. This includes sorting, selecting and sending originals (which in itself may be a major undertaking); and then providing sufficient associated information to enable the completion of the catalogue and indexing for each.
Before an image may be digitised, copyright clearance must be obtained from the copyright owner, which should be the donor or the donor’s institution for which he is authorised to sign a contract conferring to the project a licence to use the images (see Copyright below).
The permission of the patient, or subject of the image, must normally be obtained before an image acquired during the course of medical treatment can be used for any purpose other than diagnosis or treatment. It constitutes a breach of the confidential doctor-patient relationship to use the image other than for the purpose for which the patient gave permission for the picture to be taken. Certain images are exempt from this (see Ethics below).
This will be required
at a later stage, after digitisation, so that the image can be adequately
catalogued and indexed. (See Cataloguing and Indexing
below).
iii) Contract issues
In order to minimise
time spent on digitisation of images for which copyright, ethical,
cataloguing or indexing problems may later emerge, donors are sent a full
pack of documentation at an early stage. This includes:
Copyright
Image copyright, when it is concerned with Internet transmission and usage, is a complex and evolving area. The MIDRIB project has considered the area thoroughly and produced sample documentation which is shown in the annexes to this report. However, neither this nor the following discussion constitutes legal advice, and MIDRIB strongly recommends that it be used as a basis only for local development.
Commonly accepted practice in this field is still maturing, and case law is scant. All projects must therefore seek appropriate advice for their own needs, since the environment within which we are working is itself undergoing rapid change.
There is one very simple rule:
Do NOT digitise an
image if you have not obtained proper permission from the copyright owner.
However, this very
simple principle can translate into a complex and time-consuming
administrative process. Many creators of images are themselves unaware of
their rights in these cases, so ascertaining definitively whose permission
should be sought can be difficult. In addition, many teachers and
researchers have in their ‘private’ image collection slides
which they have acquired over a long period. Some they have made
themselves; some have been given to them; some have been borrowed and not
returned; some have been copied from other material and are themselves
breaches of copyright. Typically the donor is hard put to remember the
details.
There are two courses of action open at this point; take up the task of copyright tracing, or regretfully refuse all images of dubious provenance.
‘Rights tracing’ is a long and often unrewarding activity, and since the majority of medical images useful for education are not by any means unique, we do not recommend it where there are alternative solutions.
(However, the keenness of an individual to get an image digitised even though they can’t state its origin tends to show that if it could be done it would be useful. It is well worth taking note of the subject of such images to confirm their value for possible future commissioning.)
Materials to be delivered over the Internet need to consider the copyright implications of any and all of the countries in which they may be accessed. Although there have been international copyright agreements between certain groups of countries for many years, the process of making this area of the law sufficiently straightforward for the practical open provision of materials will take some time. MIDRIB therefore recommends that access is limited in the first instance to UK sites, and opened up gradually to other countries, with great care being taken over security issues.
To use an image, the copyright owner has to give his/her permission — normally in the form of a ‘licence to use’ the image for an agreed purpose. There is no need to transfer copyright ownership, and the existing owner retains full rights to use the images for all other purposes.
If the collection is to
be commercially exploited, this section would need to be reconsidered and
appropriate changes made to project documents.
Ethics
The Code of Responsible Practice for the Inclusion of Medical Images, produced with the assistance of Professor Len Doyal at St Barts and the Royal London Medical College is attached at Annex C. The conditions laid down cover pre-existing collections, and the permissions outlined within it should be obtained for all images in collections which already exist, as well as those proactively commissioned. Patients who are the subjects of old images in existing collections should be contacted, and their consent obtained; where the patient is deceased, the next of kin should be contacted.
The project cannot pass liability in this area to the image donor by accepting the images in good faith, even where they have signed a legal contract warranting and guaranteeing that the images meet our ethical specifications. For certainty, the project needs a Medical Ethics Panel including individuals with clinical, ethical and legal expertise and specialists invited for specific issues. Their main role will be to specify procedures to be undertaken in cases where the basic rules and procedures cannot be applied.
There is a strong temptation to ‘harvest’ collections that already exist, providing a wide range of high quality images for potential inclusion, often well-targeted to our main audience. However, the administrative overhead associated with obtaining appropriate ethical consent puts these collections out of reach (cf. the Wellcome’s experiences of rights tracing). In the production of a resource of any size, it will not be possible without at least one fulltime post to trace subjects of images in pre-existing collections. MIDRIB therefore recommends that such collections are not actively sought.
MIDRIB recommends an approach which concentrates immediately on pro-active collection of images: making the code of practice widely available so that images can be obtained which are in line with the project’s requirements. This also requires work to be done on lists of images required.
It is worth noting that some images have a low ethical overhead. These include ‘discards’, ECGs, etc. and so there are images that can be digitised in the short term and do not require any form of patient consent. A list is included in the ethical documentation at annex C. On the other hand, some images (such as those relating to child abuse, and to children in general) have an extremely high overhead, and MIDRIB recommends that these images be pursued later rather than sooner. The ethical issues relating to all of these are much more complex, needing to be handled with great care, and in some cases even involving legal advice and court decisions to grant consent.
Some existing collections are "special" for various reasons, and cannot be proactively replaced. (For example, high-dose X-rays which can no longer be taken, but which are extremely clear and so valuable in an educational context; and images reflecting the history of medicine.)
In these cases, the onus would be on the project to show that these images are sufficiently important to justify their inclusion. In addition, reasonable steps must be taken to locate and obtain consent from the patient or the next-of-kin. If, after all reasonable possibilities have been exhausted, it has not been possible to obtain consent, and if a judgement has been made that despite this the image is so important it should be included, it must be totally anonymised before inclusion. The Medical Ethics Panel must lay down the procedures under which a decision for or against inclusion would be made.
The project does have a direct responsibility to the patient which must be addressed, either indirectly through application of the Code by others, or directly, by contacting them and obtaining consent.
MIDRIB cannot
stress too strongly the importance of ethical compliance for the
credibility and ultimate success of a library of digitised medical images.
The implications of compliance are major, but they cannot be ignored.
Although a number of digitised collections are available now on the
Internet, it is uncertain what ethical policies are being applied.
The two-stage contract
MIDRIB recommends that donors of images sign an agreement confirming that they own the copyright of the images and that they grant the project (and its end users) a license to use their images for education and research (but NOT for publication in any other way or which would lead to commercial profit).
The full Donor Licence
Agreement (DLA) should contain accurate identification of the images whose
rights are at issue and a small print of the digitised image is the best
way of doing this. These cannot be incorporated within the agreement
without digitising the images — and this would in itself constitute a
breach of copyright without the owner’s permission, even though the
resulting files would not be made available for use by anyone. The first,
interim agreement is designed to allow this first phase to take place.
Such use includes:
It does not include:
Possible uses which we
have not considered in depth:
Together these
agreements allow:
They state that:
" The MIDRIB collection of medical images is
protected by copyright. Duplication or sale of all or part of the image
collection is not permitted, except that images may be duplicated by
authorised users for educational or research purposes either as prints or
by downloading. You are not permitted to alter any image without prior
permission from the copyright owner or to offer prints or downloaded
records, whether for sale or otherwise, to anyone who is not a member of
staff or a student of an institution authorised by MIDRIB to use the
collection. Permission for any other use must be obtained from the
copyright owners. "
The copyright owner of
the images:
(MIDRIB recommends
that in a situation where a third party contacts the project claiming to
own the copyright of an image which has been accepted claiming to be the
owner, that image will be withdrawn from the service and the two parties
will be put in touch with each other to sort it out between themselves. If
the project acquired or administered the copyright, it would be
responsible for any legal defence itself.)
The donor also states
that appropriate ethical clearance is in place for all the images, or
draws any problem images to the project’s attention.
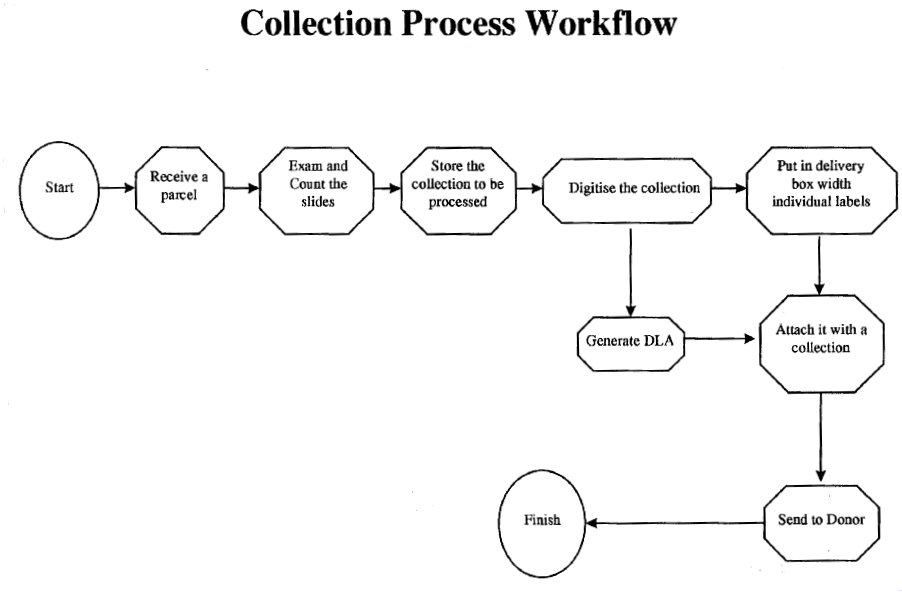
Tasks undertaken and completed in phase 1
a) MIDRIB receives Interim Donor License (IDL)
and images
After receiving the
documentation outlined above, the donor selects slides from their
collection (or acquires them according to the project’s commission)
and sends them, with a completed interim license, to the project for
processing. The IDL allows the project to digitise, anonymise if
necessary, but not to use or manipulate the images in any other way.
i) Slides examined and counted
On receipt of the package, the slides are counted and their physical condition assessed: this information is entered into the workflow system. A receipt is sent and any damage reported.
Assessment of physical condition has two purposes:
1) A duty of care to the donor. It also protects the project from allegation of damage since it will be possible to say that certain images were or were not damaged on arrival. If any images are damaged on arrival, the donor must be immediately informed
2) Some slides may need
to be cleaned and /or remounted before digitisation and this work must be
scheduled.
ii) Collection and IDL stored
to be processed
The Interim Donor License is filed for future reference. Slides are placed in a secure location, details of which are entered on the workflow system.
MIDRIB recommends that
care of donors’ materials must be seen to be of a very high and
professional standard, since they are generally perceived by the owners to
be valuable and irreplaceable resources. Obtaining a good reputation
in this area will ease negotiations with future donors. On the other
hand, explaining to the donor that their slides are useful to the
resource, but can be easily reacquired from other sources, may be felt to
be insulting to a donor who is contributing in order to obtain a measure
of recognition from the healthcare community.
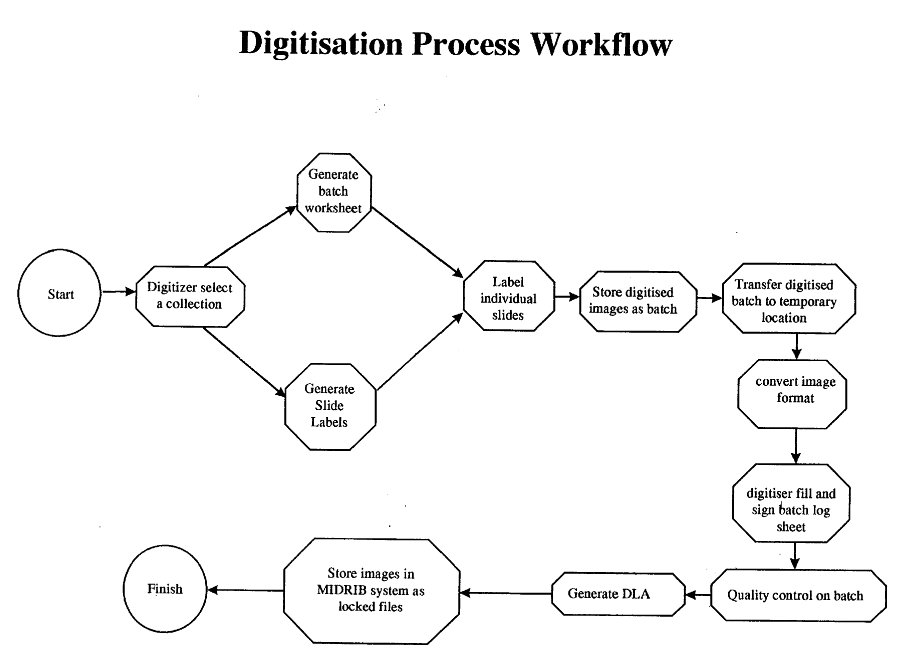
b) Digitisation
i) Digitiser selects
collection
The digitiser goes to the workflow system to see what collections are currently awaiting processing. This information is generated from a) above. ii) System generates:
This gives information on the exact slides to be digitised, their location and physical condition. A "batch" consists of the number of slides that will be handled in one pass of the digitisation equipment (e.g. one carousel) and associated quality assurance slides. A maximum is seventy slides in the carousel of a Kodak Digital Conversion System. A minimum could be a single slide in certain circumstances.
Once a batch worksheet has been generated, the batch should be completed that day.
Before slides are
mounted into the carousel, the system generates sticky "batch"
labels. These are bar-coded, with numbers reflecting the donor I.D.,
collection I.D., batch I.D. and the position of the slide in the carousel.
These, together provide the ingredients for a unique file name with which
the project will subsequently identify the image.
iii) Labels applied to individual slides
These labels are
carefully applied to the slides as they are loaded into the carousel to
prevent errors of misidentification. The digitising equipment cannot,
naturally, read the label as it digitises, and will therefore generate the
number element of the file name from a slide’s location in the
carousel. Locating the label on the slide must be done with care, since it
further identifies the orientation of the slide. Slides are checked to
make sure they are clean and dust free. iv)
Batch digitised
The carousel is mounted
into the digitisation equipment, and the digitisation process takes place
(approximately one hour per full carousel).
v) Store digitised images as batch
The files generated
during the digitisation process on the Kodak digitiser or similar
equipment cannot remain there, since the storage space is insufficient.
They are kept together, as a batch, for future identification.
vi) Transfer digitised batch to temporary location
These files are
transferred to a temporary location on the project’s raid array.
c) Convert image format
The files are converted
to PNG from the native format of the digitising equipment, usually TIF.
(PNG is a royalty-free lossless compressed graphics file format - see
http://www.boutel.com./boutell.pngfor
PNG specification)
d) Digitiser fills and signs batch log
sheet
At this point the
substantive work of the batch is completed, and the digitiser makes any
notes needed, signs the batch log sheet to indicate its successful
completion, and returns the slides to temporary storage.
e) Quality control on batch
A number of quality
control slides are inserted at specific locations in the carousel, and
these must be checked before the images are released for further
processing.
f) Generate Donor License Agreement ‘s
Annex
Before the next phase
of work on the images can begin, the project must receive from the donor a
full Donor License Agreement. Annex B of this agreement contains printed
representation of the images (which have digitised as described above)
covered by it. These sheets are now generated by the workflow .
g) Generate Image Description forms
At the same time, an
individual sheet for each image is generated for the donor to use in
supplying textual information relating to the image. (see Annex B of this
report).
h) Store images in MIDRIB system as locked
files
The images are locked
in the project system software, so that no further work can take place
before appropriate permissions have been obtained. This also contributes
to system security.
i) Pack slides (still labelled) for return
The slides, in their
cleaned and remounted state and with project labels still attached, are
carefully packed by the digitiser for return to the donor. Leaving the
labels on the slides facilitates any follow-up that may be necessary and
enables the donor to easily identify an individual slide using the project’s
own code.
The packed slides are returned to the donor, together with annex B of the DLA and the full Donor License Agreement, together with copies of the information-gathering forms. Donors also receive guidelines on completing the information-gathering forms.
The content coordinator who conducted the negotiations subsequently contacts the donor to ensure that originals and paperwork have been received, and to check that there are no problems with completion.
a) Donor receives originals, Donor Licence
Agreement (DLA) with appendices & Image Data (ID) forms with
guidelines
The donor receives the
originals (s)he sent to the project, cleaned and remounted if necessary
and with project labels linking originals to digitised versions held in
waiting for the additional catalogue information to be received. These are
returned with the DLA and its appendices and ID forms.
b) Donor completes forms according to guidelines
and returns them to the project
The descriptive information that is available to enable retrieval of each project image will vary in its completeness. Sometimes it may be a single line caption, other times it may consist of a descriptive paragraph or two. The specimen MIDRIB Guidelines to Contributors outline the format and content of the annotations that MIDRIB recommends that the project obtains. In real life there will be deviation from such guidelines, and no time or expertise to enhance the descriptions. As much information as possible must be sought directly from image donors at this stage. Enhancing descriptions retrospectively is unlikely to be an attractive or practical option, though it should not be excluded in selected cases.
Full description of the image is essential, so that users can successfully retrieve images from the database. In addition, meaningful descriptions enable the user to evaluate how useful an image may be for their own purposes. Importantly, subject indexing is derived from, and therefore limited by, the amount of descriptive text that accompanies each image, as supplied by the image donors.
It may be advantageous
to provide a facility for the donor to supply descriptions on disk or
online.
c) Information entered from the ID forms into
the database — tied to digitised image by bar code on form
The donor completes the
full Donor License Agreement and returns it to the project. They may then
send information forms as and when they fill them in. When these are
received by the project, the information contained is entered into the
database to provide the catalogue. Although donors will be working to
guidelines, it should be noted that their total compliance with our
expected format cannot be guaranteed, and some interpretation and
flexibility may be required at the time of data entry.
d) Data entry Quality control
A specified percentage
of data entered is checked by another member of staff against the
information forms. In addition to content accuracy, matching of
information against image must also be checked to ensure that the barcode
system has not failed due to operator error. Checking is particularly
important in situations where the information has had to be interpreted to
fit, or where handwriting is a problem.
e) Information used to index the image
appropriately in MeSH.
An important way to empower the user is by providing a sufficiently indexed collection. Each of the elements of information supplied by the donors, and especially the descriptive text and suggested keywords, is used to generate additional indexing terms to aid subsequent search and retrieval.
MIDRIB recommends the use of the MeSH thesaurus (the US National Library of Medicine’s Medical Subject Headings) for subject indexing. No other scheme is currently as widely in use in the digital library arena. MeSH is the indexing system with which most learners, academics, librarians and curriculum developers may be familiar through their experience of searching Medline and other medical resources. Use of MeSH will allow interoperability with a range of related services, notably OMNI (Organising Medical Networked Information). MeSH is also a key element of the UMLS (Unified Medical Language System) metathesaurus.
MIDRIB recommends that the indexer has the facility to explore MeSH online. The MeSH thesaurus may be implemented so that the indexer simply clicks to select a term, avoiding the need to cut and paste, or type in terms directly. The use of UMLS to facilitate computer-assisted indexing may be explored.
Extensive in-depth,
multi-faceted, specific indexing, to the level of discrete MeSH tree
locations, can enhance the chances of relevant images being retrieved in a
consistent manner. Such in-depth indexing would be essential, for
instance, if browsing and search features were to be implemented based on
the hierarchical tree structures of the MeSH thesaurus.
f) Indexing Quality control
Quality control
procedures to ensure that the right text accompanies the right image
before the indexer begins the task of subject indexing must be in place.
As with all the information that accompanies an image, accuracy and
completeness of indexing is important. Incompletely indexed fields may be
appropriately flagged. To test procedures effectively, records may be
sampled frequently to begin with, with a reduction in frequency over time.
That way, recurring indexing issues can be identified early and solutions
sought. Regular reviews of progress in achieving quality standards should
be made.
g) Search and retrieval issues
Detailed descriptions
as provided by the image donors and in-depth multi-faceted subject
indexing as described here, would form the foundation for subsequent
effective search and retrieval. MIDRIB recommends that all textual
information is fully searchable, alongside MeSH subject indexing terms.
A set of default search options should be available to the user. A choice of search interfaces can be made available, with greater flexibility built in to an enhanced search interface.
Free text searching
will allow :
Automatic stemming / automatic pluralisation / synonym identification may be supported if possible.
MIDRIB recommends that browsing and searching using MeSH should be supported.
Browsing offers an
effective approach to retrieval as it allows exploration in context.
Provided appropriate technical expertise is available, it may be possible
to design browsing features so that :
In addition to
browsing, it should be possible to search for known MeSH terms directly.
Provided appropriate technical expertise is available, it may be possible
to guide the user to existing MeSH headings. The MeSH scope notes, if
implemented as a glossary, may provide additional help.
Even with well-defined
topics, such as a disease or a drug, multiple synonyms may be used to
describe a single concept. To rely exclusively on the text description
provided by donors and the MeSH headings assigned by indexers can be
restrictive in ensuring retrieval of potentially relevant material that
may be present in the database. Provided appropriate technical expertise
is available, it may be possible to implement a mapping feature, using
UMLS to translate search queries to MeSH. Search queries mapped through
UMLS will employ the richness of the constituent UMLS thesauri.
ii) Interoperability
MIDRIB recommends that developments in the metadata field are monitored to ensure that interoperability with related datasets will be possible.
The Dublin Core metadata set, Educom’s Instructional Management
Systems (IMS) metadata specification for educational materials, which
builds on and incorporates the Dublin Core metadata elements and XML/RDF
for the exchange of metadata are recommended for close monitoring. The
work of CIMI (Consortium for the Computer interchange of Museum
Information) on distributed searching of museum and bibliographic
information, and especially with regard to international Z39.50
interoperability, is of key interest, as are protocols such as WHOIS++.
Practical examples of the application of metadata standards are being
documented by TASI (Technical Advisory Service for Images, http://www.tasi.ac.uk/).
a) Donor signs both copies of Donor Licence
Agreement (DLA) and each page of appendix B, and returns one copy to the
project
In order for the images
to be made available to authorised users on CDs or via the Internet, the
full DLA must be completed. One copy is kept by the project and one by the
donor; each supplemented by an appendix containing printed representations
of the images covered by the agreement. Each page of this appendix must be
signed by the donor.
b) Donor states in DLA that our ethical
standards are met and that copies of patient consent forms will be sent;
or outlines the ethical situation for each image where the
situation differs
The ethical issues must be clearly addressed for each image. It is not sufficient for the project to take the donor’s word that there are no ethical problems associated with the project’s intended use of the image, since the project itself has a responsibility to the patient. As a result, copies of any available consent forms must be received and stored for all images whilst they are part of the resource. These are normally received prior to starting the onerous work of indexing and cataloguing.
For some images it may
be difficult to locate such permissions, for example in the case of a
hospital, which has since closed down. In this situation it may also not
be possible to trace a patient to obtain retrospective consent. These may
be referred to the project’s Medical Ethics Panel.
c) All images subject to ethical doubt must be
approved for inclusion via the procedures laid down by the project’s
Medical Ethics Panel before inclusion takes place.
If the donor believes
than an image is of such value that it should be included in the resource
despite difficulties in demonstrating patient consent, they should make a
statement to that effect. This should outline the nature of the image’s
value and giving reasons why a replacement cannot be proactively
commissioned where ethical standards can be controlled. The Medical Ethics
Panel will lay down procedures to be followed in such situations. MIDRIB
recommends that the Medical Ethics Panel should include:
There should be no
images for which neither clear proof of patient consent, or cases for
inclusion as given above, have been received.
d) In an unforeseen circumstance,
the images will be laid before the Medical Ethics Panel and an appropriate
procedure devised.
Where an ethically unclear situation arises (in relation to an image or images which are considered to be of prime importance for the resource) the case itself is laid before the ethical panel who have responsibility for devising an appropriate procedure under which a decision on its inclusion can be taken. The resulting procedure(s) will be documented and added to the existing special occasion procedures to be used in any similar situations in the future.
No images without the patient’s consent may be included in the resource before they have been cleared by the project’s documented procedures. If such an image does not obtain clearance, any electronic or printed copies of it should be removed from the project’s files and a record kept for future reference by the ethical panel.
The only exception to
this process is where the Medical Ethics Panel has agreed special
procedures that might, for example, apply to historical images of
particular importance.
e) CD of images plus catalogue and
indexing information returned to donor
Once phase 3 is complete, MIDRIB suggests that the project make up a CD for the donor comprising a full searchable set of their images complete with catalogue and indexing information. Ideally this should utilise the project’s existing software. The costs of doing this need to be assessed. There is no formal commitment in the DLA to provide a copy of their slides/images on CD to the donor. However, if technically and financially viable it is desirable, since it fulfils two purposes:
(i) To enable the donor to check the quality of the digitised images, and the accuracy of data entry and indexing.
(ii) To provide the
donor with an immediate tangible benefit of contributing to the resource
f) Where licensing and ethical conditions
are met, images are made available through the resource after 30 days.
The donor has 30 days after receiving the returned slides (and CD, if this is sent) to check it and to send any corrections to the project for incorporation. If this time expires without any corrections being received, the images and associated information will become a part of the resource.
When corrections are
received, the project may decide to allow the donor an opportunity to
request a new CD for further checking. However, it should be noted that
this could snowball into a major overhead, and care should be taken that
its main purpose really is checking rather than provision of an image CD!
If this course is not taken, the normal Quality Assurance procedures can
be deemed to be adequate, and the images and associated information are
released on the Web and made available for users to search and download as
part of the secure resource.
5 Phase 5 — User
Licensing
a) Unauthorised use
MIDRIB has taken the view that completely eliminating copyright breaches and ethical abuse of images is beyond the reach of a project of this nature. We therefore recommend strategies based on making individuals responsible for their own actions throughout, minimising liability to the project itself. This approach informs the agreements discussed above and given in annexes A and B, and theEthical Code shown in Annex C. However, there does remain at the least a moral duty for the project to do whatever it reasonably can to prevent unauthorised access and use to images.
There are a number of reasons for this. Firstly, there is a duty of care implied in patient consent, which it would be unacceptable to avoid. Secondly, it would be undesirable for gruesome and distressing images to be widely and openly available on the Internet. It would be unfortunate for children to accidentally access them, for example, and there are other, even less desirable, deliberate usages which could damage the project’s reputation as a serious medical education resource. Finally, there is the personal damage which open and disreputable use of images could do to the patient or subject. There are laws governing the dissemination, for example, of medical images of children in any medium, which must be complied with.
The project cannot control the use of an image once it has been downloaded, so care must be taken to ensure that access does not fall into the hands of users who may abuse the resource or any part of it. The project must also be very careful to ensure that it is not legally liable for such abuse, since the penalties may be severe.
MIDRIB therefore
recommends that images be made available only to recognised institutions
and that password access must be enforced. We hope that administration of
such a system will be greatly improved with the implementation and further
development of ATHENS, and recommend that projects follow up any
developments in the area of Internet security as a matter of priority in
their own project development.
b) Institutions register one or two
authorised individuals with the project who will be responsible for
registering their staff and students to use the resource
There are approximately 800,000 potential users of a digital medical image library in the UK. The administration of this volume of usernames and passwords, let alone the verification of professional standing required (see above) represents an insupportable administrative overhead. However, as stated above, these elements are essential.
MIDRIB recommends that individual registration of authorised users is carried out in each registered institution by their authorised personnel.
One or two individuals
would be proposed by the registering institution, and given any training
needed by the project. These individuals would come from central
departments who routinely deal with staff and students in servicing their
educational and research needs, for example the computer unit, library or
registry. They will be in a position to verify the appropriateness of the
application for a password, and further to inform the project once this
period has passed (for example when students finish their courses).
c) Terms and conditions of use
Use of the resource
must comply with the project’s undertakings to the image donor and by
extension to the patient subject of the images. This is outlined in Phase
1. The institution is responsible for ensuring this, and access may be
terminated by the project at its discretion if breaches are found to have
taken place.
d) Levels of access
There are levels of sensitivity within the potential material for a medical image resource. This can be seen very clearly if one considers the issues involved in, for example, an image of nettle rash as against an image showing the results of deliberate harm of a child. Some images are too sensitive to be widely available even within the structures laid down above, and should not be committed to the Internet until such time as the security of the Internet resources can be absolutely guaranteed.
As this level of certainty may be many years away, images of child abuse or genital deformation, etc., should be handled by the project as they would handle nitroglycerine. It is unfortunate that these images are important, and medical practitioners in certain fields need to be as familiar with them as they are with the more routine materials. The sensitivity surrounding them makes them particularly difficult to come by for legitimate users. There may therefore be good reasons for making them part of the project’s work.
MIDRIB recommends that particularly sensitive materials be distributed only on CD initially and that these CDs be placed in the reserve collection of registered institution’s libraries. Should an institution’s access be terminated, all such CDs must be returned to the project. The project’s internal processes must be carefully examined; security and suitability of project staff to view and manipulate such images must be formally cleared.
The project may wish to
make some non-controversial images available more widely for the purposes
of patient education. These could include, for example, pictures of
medical equipment with associated information on their use; home
diagnostic material ("should my GP check out this mole for possible
skin cancer?"); poisonous plants; information on controlled substance
or alcohol abuse; etc. Such images and information must clearly be
selected with great care — copyright and ethical standards must apply
with even greater stringency, and there is a duty of care towards the
public. However, once an appropriate selection has been made, a number of
records flagged for open access may be made available.
e) Termination of access
Rights of access may be
terminated by either the registered institution or the project. The
project must have the right to terminate access by any institution staff
or students if the institution has demonstrated that it cannot guarantee
appropriate use of the resource, and should be able to do this at its own
discretion. Access may be reinstated once the institution proves that new
procedures are in place.
6 Image review
MIDRIB recommends that
the project review the content of its resource continually, to ensure that
it is relevant and sufficient for its users’ requirements. Detailed
peer review of images prior to inclusion would undoubtedly be desirable,
if the project has sufficient resources. However, MIDRIB feels that most
projects will be unable to support the administrative overhead and
possible consultancy fees that this would entail, and so here we outline a
number of alternative strategies:
a) Logging usage patterns
The way in which the
resource itself is actually used is the best guide to the most and least
useful images and groups of images. All access should be logged, and
records kept of the number of hits, downloads, etc. of each image.
b) Deleting unused images after a period
When it becomes
apparent that an image is never called for, the project may decide to
transfer it to off-line storage in order to make space for more in-demand
materials. However, MIDRIB recommends that this decision be deferred
for a year from the date of inclusion since access may be limited to
certain periods of the teaching cycle. Granted, it is unlikely that these
would harmonise across the whole of UK medical education, but disk space
is relatively cheap and it would be a shame to have removed resources,
which later turn out to be required after all.
c) Capturing unsuccessful searches for
proactive collection building
Particularly in the early stages of building the library, users will from time to time enter a search, which is unsuccessful. These searches should be considered as requests, and images satisfying them should be added to the list of images to be proactively sought. This is a particularly powerful way of ensuring that the users’ needs are met.
It may also demonstrate
a lack of familiarity with online searching (for example in first-year
students), and where regular errors appear the project may wish to
consider adding tailored searching help to the resource.
d) Online comment and submission
Academics are not shy of debate, and this characteristic can be used to positive effect in the honing of a resource of this kind. By providing a facility for online comment on an image (e.g. "this slide shows an unusual combination of characteristics — I have a much clearer series of images") it will be possible to provide not only the originator’s comments, but elements of an ongoing discussion possibly leading to the improvement of image quality and usefulness. Such discussion will also widen student appreciation of the interpretation of medical images.
Such a facility would require some monitoring on the part of the project’s team, and we recommend that the facility for online submissions of text or images be built in such a way that an email alert is also received.
It should be noted
that the volume of this kind of exchange will probably not become very
significant until the resource itself becomes established and recognised
in the field.
Annex A — Interim License [IDL] &
covering letter
MIDRIB Interim Donor License
1 The parties to this interim agreement are ...........................................…..............................
(the ‘Copyright Owner’) whose address is ........................................................................... ................................................................................................................................................................................................................................................................................................
and St George’s Hospital Medical School of Cranmer Terrace, London, SW17 0RE (‘MIDRIB’).
2 The Copyright Owner shall promptly at its expense provide MIDRIB with a copy of their collection of medical images (the ‘Collection’) in an agreed format. MIDRIB may, if necessary, at its expense convert all or part of the Collection to a format suitable for its operations.
3 The Copyright Owner grants to MIDRIB a non-exclusive licence, subject to the terms and conditions of the full agreement to be signed later (the ‘Agreement’), to process the Collection (including conversion into digitised machine-readable form by MIDRIB) for the purpose of creating printed sheets of images which will form Appendix B of the Agreement. These digitised images will form the basis of the work to be undertaken as outlined in clause 3 of the Agreement.
4 MIDRIB undertakes the work of digitising the Collection on the understanding that the Copyright Owner has read the Agreement and will be supplying any other documentation required and will sign the Agreement on receipt of the sheets of image prints. If the Copyright Owner does not sign the Agreement, the digitised images will be erased from the MIDRIB database and the Collection returned.
5 MIDRIB will take reasonable care of the Collection, but accepts no responsibility for loss or damage of the Collection however caused.
6 Where the copyright owner is NOT the organisation named above, please tick one of the boxes below to identify who is the copyright owner:
~ the consultant or clinician [ ]
~ the photographer [ ]
Signed for and on behalf of the Copyright Owner
by:
.................................................................................................
Date : .......................
Signed for and on behalf of MIDRIB
by:
.................................................................................................
Date: .......................
Covering Letter with IDL
[Contact name] Draft - revised 5/6/1998 copyrit9.doc
[Contact address]
[Contact address]
[Contact address]
[Contact address]
[Contact address]
Date
Dear [contact]
Medical Images Digitised Reference Image
Bank (‘MIDRIB’)
Thank you for your interest in contributing medical images to MIDRIB - an eLib project currently funded by JISC. To enable us include your images you must give us a licence to do so. When you do this you retain your copyright in the images and can continue to use them, for example may still give others non-exclusive rights to use the same images, such as people who wish to use them in books or for commercial applications.
The enclosed paperwork is necessary to protect both you
and the MIDRIB project team. It aims to clarify how your images may be
used by the MIDRIB and by authorised users of the MIDRIB service.
MIDRIB wishes to develop a service which will allow staff and
students of registered institutions to access, display, retrieve and print
images from the collection via the Internet and by other means for
private, educational or research or diagnostic purposes. Users will not be
permitted by MIDRIB to use any part of your collection for commercial
purposes, including publication in books and magazines - requests for this
use must be made to you as the copyright owner.
This permits MIDRIB to digitise your collection, but not to make it available to users at this stage. The copyright holder is normally the organisation (medical school or NHS Trust). but is some cases the clinician or the photographer. You should sign the interim licence (after having read the other documents) and return it to us with your images. We cannot to proceed until we receive this.
Currently MIDRIB is only asking for 35mm slides.
When we have digitised the images, we will return the originals to you together with a licensing agreement containing printed copies of the selected images. Also included will be one sheet for each image on which you can provide the information describing the image, which will enable us to catalogue it on the MIDRIB database. MIDRIB may need to edit these descriptions of the images when adding them to the system to, for example, provide a more consistent style which will assist MIDRIB users when searching for suitable images.
You sign this agreement and return it to us with the completed image
forms (and patient permissions where these are required). Later a copy of
the completed agreement will be returned to you.
With some images of people we may also require permission forms
signed by the patient for medical ethical reasons. But to keep things
simple at this stage, MIDRIB are concentrating on images where patient
permissions are NOT necessary. Enclosed is a list of the images where
patient permissions are NOT required according to our independent
advisors.
The finalised documentation will be kept by the MIDRIB team in an
archive at St George’s Hospital Medical School.
Please get in touch if you have any queries. And, finally,
thank you for coming to us with your image collection.
Yours sincerely
MIDRIB Production Manager
Enc: List of images for which patient permissions are NOT required.
MIDRIB Interim Donor Licence
MIDRIB Donor License Agreement
Guidelines for delivery of your collection to MIDRIB
Medical Ethics
The following list of images do NOT require signed patient permission forms (providing there are no labels identifying the patient directly or indirectly). This list is consistent with the MIDRIB Code of Responsible Practice.
MIDRIB will ensure that any identifiers on the images are removed or erased when they are digitised, so that the images displayed on the MIDRIB service to authorised users are acceptable.
Annex B — Donor License Agreement
[DLA] & notes
MIDRIB Donor License Agreement [ Number ]
An AGREEMENT made this ...... day of .............................. 19 .....
1 The parties to this agreement (the ‘Agreement’) are ............................................................ (the ‘Copyright Owner’), whose address is .......................................................................... ....................................................................................................................…........................
................................................................................................................................................
and St George’s Hospital Medical School of Cranmer Terrace, London, SW17 0RE (‘MIDRIB’).
2 In this Agreement, ‘Collection’ means the collection of images as described in Appendix B, whether in machine readable or other form, and any part thereof including data that has been included with the images by the Copyright Owner. A list of other definitions of the terms used in this Agreement given in Appendix A.
Licence
3 The Copyright Owner grants to MIDRIB a non-exclusive licence, subject to the terms and conditions of this Agreement, to use the Collection in the following ways:
a To process the Collection (including conversion into machine-readable form) for the purpose of creating searchable and displayable files based on the Collection and to modify the image where necessary to disguise the identity of a patient and to load these files onto the MIDRIB Service.
b To permit Authorised Users to perform searches and/or to download and/or to print the results of searches and to reproduce all or part of the Collection in the form of non-electronic visually perceptible form, such as Prints. All subsequent access to and use of such reproduced material will be for Educational and Research Purposes of Authorised Users and such downloaded, printed or reproduced material may not be offered, whether for sale or not, by MIDRIB to anyone who is not an Authorised User.
c To make copies of any machine-readable version of the Collection and updates for back-up purposes for the MIDRIB Service.
d To permit Authorised Users to download all or part of the Collection, for Educational and Research Purposes by themselves and other Authorised Users.
e To reproduce, distribute or publicly display images from the
Collection in a manner compatible with the Educational and Research
Purposes and for publicity associated with the MIDRIB Service.
Distribution of the Collection to Authorised Users may be on compact discs
over computer networks or other appropriate means.
4 This licence is granted on condition that MIDRIB draws the
following notice to the attention of Authorised Users:
" The MIDRIB collection of medical images is protected by
copyright. Duplication or sale of all or part of the image collection is
not permitted, except that images may be duplicated by authorised users
for educational or research purposes either as prints or by downloading.
You are not permitted to alter any image without prior permission from the
copyright owner or to offer prints or downloaded records, whether for sale
or otherwise, to anyone who is not a member of staff or a student of an
institution authorised by MIDRIB to use the collection. Permission for any
other use must be obtained from the copyright owners. "
5 MIDRIB will take reasonable measures to prevent unauthorised
access to, duplication of, or distribution of the Collection.
Copyright ownership
6 The Copyright Owner represents and warrants that it is the sole owner of the copyright in the Collection and the images within it, or that it is duly licensed or authorised to grant the rights it offers in this Agreement in respect of the copyright material contained in the Collection, and that the Collection used as contemplated in this Agreement will not infringe any copyright or other proprietary or intellectual property rights, including moral rights, of any natural or legal person. The Copyright Owner shall indemnify and hold MIDRIB harmless from and against any loss, damage, cost, liability or expense (including reasonable legal and professional fees) arising out of any actual or alleged infringement of such rights. MIDRIB shall inform the Copyright Owner of any such infringement or suspected or threatened infringement. This indemnity shall survive the termination of this Agreement for any reason. This indemnity does not apply to any data added by MIDRIB to the Collection.
7 MIDRIB acknowledges that copyright in this Collection is held by the Copyright Owner and that no transfer of ownership of copyright is conveyed by this Agreement. The copyright in any additional data added by MIDRIB to the Collection, and any search software, user guides and documentation prepared by MIDRIB to assist Authorised Users to use the Collection shall belong to MIDRIB and any other parties that MIDRIB may choose to enter into an agreement with to produce such materials.
The Collection
8 The Copyright Owner further represents and warrants to MIDRIB that the Collection will not contravene any laws, including but not limited to the laws of libel, defamation and contempt of court (or concepts approximating thereto). The Copyright Owner shall indemnify and hold MIDRIB harmless from and against any loss, damage, cost, liability or expense (including reasonable legal and professional fees) arising out of any illegality or alleged illegality. Either party shall promptly inform the other of any illegality or alleged illegality upon the party becoming aware of the same. This indemnity shall survive the termination of this Agreement for any reason. This indemnity shall not apply to any data added by MIDRIB to the Collection.
9 The Copyright Owner does not warrant or guarantee the Collection in terms of the comprehensiveness, accuracy, reliability or otherwise of its contents.
Royalties and charges
10 No royalties shall be paid by MIDRIB to the Copyright Owner for use of the Collection, including for running the MIDRIB Service, for the purposes of marketing, promoting, demonstrating or testing the MIDRIB Service, or for training of Authorised Users or for Educational or Research Purposes by Authorised Users.
11 MIDRIB shall at its sole discretion decide what charges, if any, it shall make to Authorised Users of the MIDRIB Service. The MIDRIB Service is non-profit making and money collected from Authorised Users will be used to run or enhance the Service.
Term of the Agreement
12 This Agreement shall take effect on execution hereof and shall continue for ten years, when a renewal of the licence for further periods of ten years may be assumed unless either party requests in writing that it be allowed to lapse.
Termination
13 In addition to any remedy, either party may terminate this Agreement immediately without further obligation in the event of:
a any breach of this Agreement which cannot be remedied or is not remedied within thirty (30) days of the party in breach being requested to do so by any other party;
b any party making any composition with or assignment for the benefit of its creditors;
c any resolution being passed or petition being presented to wind up any of the parties business (otherwise than for reconstruction or amalgamation) or a receiver being appointed of the whole or part of the party’s assets.
General
14 This Agreement shall be governed by English Law.
15 Neither of the parties may transfer their rights or duties under this Agreement without the prior written consent of the other, except that MIDRIB may transfer administration of the MIDRIB Service from St George’s Hospital Medical School to an appropriate organisation in which case the rights and duties of St George’s Hospital Medical School under this Agreement will be novated to this organisation.
16 Neither party shall be under any liability for any loss or for any failure to perform any obligation under this Agreement due to causes beyond their control including, but without limitation, industrial disputes of whatever nature, acts of God, hostilities, force majeure or any circumstances which they could not reasonably foresee and provide against.
17 All matters contained in this Agreement and any associated information and documentation shall be treated as confidential by the parties unless such matters are or become within the public domain even after the termination of the Agreement.
Signed for and on behalf of the Copyright Owner
by: .................................................................................................
Signed for and on behalf of MIDRIB
by:
.................................................................................................
Appendices attached:
Appendix A List of definitions
Appendix B List of images contained in the Collection Appendix
A : List of definitions.
In this Agreement, the following expressions shall have the following meanings in context:
‘Authorised User’ means staff or students of institutions authorised by MIDRIB to use the MIDRIB Service of which the Collection may form a part.
‘Copyright Owner’ means the owner of copyright in the Collection or someone authorised to grant such a license by the copyright holder(s).
‘Display’ means the output, in part or in full, of Records retrieved in a search which are displayed on the screen of any terminal, workstation or personal computer used by an Authorised User.
‘Educational and Research Purposes’ includes the incorporation of images in teaching materials (to be used by institutions registered to use the MIDRIB Service), creation of teaching sets of images, atlases, direct use by students and incorporation in students’ submitted work and includes research use for reference purposes by a researcher (working for an institution registered to use the MIDRIB Service), patient diagnosis and equivalent activities approved by MIDRIB.
‘Download’ means to transfer data in any form from the MIDRIB Service to a computer storage device or peripheral or computer so that it survives an individual search session.
‘MIDRIB Service’ or the ‘Service’ means the Medical Images Digitised Reference Image Bank which is a computer based system offered to Authorised Users administered by St George’s Hospital Medical School (or such alternative organisation appointed to succeed them in administering the Service) and is currently funded by JISC as an eLib project.
‘Print’ means the output, in part or in full, of a Record retrieved in a search and which is either printed on a printing device that is connected Authorised Users’ workstations or terminals or on centralised printers of institutions registered to use the MIDRIB Service.
‘Record’ means a complete machine-readable unit of the
Collection that represents a single image, including all fields containing
data primarily associated with that image.
Appendix B : List of images contained in the
Collection
Annex C — Code of Responsible Practice
for the Inclusion of Medical Images in MIDRIB
The Code of Responsible Practice for the Inclusion of Medical Images in MIDRIB
version 8- DRAFT
Introduction
This policy outlines why and how the confidentiality of patients should be respected by staff involved in the creation the Medical Images Digitised Reference Image Bank (‘MIDRIB’). As well as respecting the ethical and legal rights of patients, the policy protects staff and image donors from potential accusations of the wrongful use of images.
All ‘medical images ’ used by MIDRIB are subject to this policy irrespective of who produces the image or record or owns the material or equipment on which they are produced. Those providing images for MIDRIB must only submit images which have been obtained by processes which conform to this policy - breach could lead to legal action. No medical image known to be in breach of this policy should be used by MIDRIB or their agents and it is the responsibility of everyone to obtain appropriate evidence of conformity to this policy.
What is a ‘Medical Image’?
In the context of this Code, ‘medical images’
(or ‘illustrative clinical records’ or ‘clinical
illustrations’) may depict any physical characteristic in any media.
For example, they include photographs, artists’ drawings or
paintings, cine films, video tape recordings, or audio tape-recordings
where the patient’s voice can be recognised, or any images, forms or
models of any part of the patient by which personal information can be
gained, even remotely.
Consent — General Policy
Patients should give their written consent for information concerning their condition and treatment to be used for any purpose other than their own treatment - this includes medical images. Patients should give consent based on information about what particular images are involved and the potential uses to which they may be put — for example, education, research or publication. Such information must be recorded on the consent form and must be signed by the patient AND the responsible clinician. The original signed form should be kept in the patient’s clinical record and a copy kept by the relevant department of medical illustration.
Trusts will have different consent forms concerning medical images. A signed consent form of a Trust is inadequate to authorise their use in MIDRIB. Patients should read the MIDRIB patient information sheet and sign its associated consent form. These signed consent forms — the Trust’s and MIDRIB’s — should be kept in the patient’s clinical record. Patients should be offered copies to keep and copies kept on file by the relevant department of medical illustration. In addition a copy must sent to MIDRIB, together with the medical images, to keep on file.
Confidentiality is paramount in the creation and use of medical images. Where patients have given informed consent in writing, an appropriate label should be attached to the photographs or other medical images - such as "Authorised Use for Education/Research" or "Authorised Use for Publication". One copy of these labelled medical images must be kept in the patient’s records together with the signed consent form authorising their creation. Ordinarily, all other records should be labelled as: "To be Used for Diagnosis and Treatment ONLY".
Failure to obtain a patient’s consent, where this is required,
is a breach of this policy regardless of the professional position of
whoever has made the medical image.
(A) When consent is NOT required
Discarded human tissue :
It is legally and professionally acceptable to use medical images of discarded tissue from surgical procedures for the purposes of teaching and research without obtaining the consent of the patient from whom the tissue was derived. This assumes that there is no way in which the appearance or labelling of such tissue can be employed to identify the patient from whom it derives. The same principle holds for the use of such discarded tissue in illustrative medical records, including use in MIDRIB.
Diagnostic Images (such as radiographs, scans, or related transparencies or prints) :
These are subject to this policy, but do NOT require a patient’s consent PROVIDING all the patient’s identifiers have been permanently removed. A list of diagnostic images which may it into this category is attached in Annex A. When required the MIDRIB team will erase or remove patient identifiers from diagnostic images during the digitising and cataloguing processes.
However, where diagnostic images remain labelled in such a way that
can identify the patient directly or indirectly, then the patient’s
written consent is still required. Indeed where it is practical to obtain
the patient’s consent, this should be sought.
Consent in Exceptional Cases
(B) Adult patients who are unable to give informed consent
When prior consent is not possible, it is acceptable to gain consent
for such use after the patient regains consciousness, but illustration
departments should only release medical images after written consent has
been obtained and the patient has been given information about their
intended use. This includes specific permission for the use by MIDRIB on
an appropriate form and the patient has read the relevant information
sheet.
Where patients do regain consciousness, illustration departments
should not release medical images until consent is obtained in writing in
the usual way.
Permission for use by MIDRIB must be further obtained on the
appropriate form after the relative has read the information sheet.
Equally all should images should be referred to and approved by the MIDRIB
Medical Ethics Panel (see E below). Medical illustration departments
should not release medical images of unconscious patients until written
consent of the patient or relative has been given.
Where patients do regain competence, medical illustration
departments should not release medical images until consent is obtained in
writing in the usual way (described in 1 above).
All these medical images should be anonymised and, where this is
impossible, should ONLY be used for therapeutic purposes. Publication of
such anonymised images should only occur after obtaining specific legal
advice from the Legal Liaison Department of the Trust involved that the
appropriate processes have been followed. Permission for use by MIDRIB
must be further obtained on the appropriate form after the relative has
read the information sheet (see E below).
(C) Deceased patients
If a patient dies without giving consent, this material may be retained until a relative can be sensitively approached. Providing the patient cannot be identified in the medical images, and providing a close relative (if available) gives written consent, then such material can be used for ‘medical education and research purposes’. However medical images of deceased patient must also be anonymised - where this cannot be done, they should not be used for these purposes.
Specific written permission of the use of medical images by MIDRIB must be obtained from the close relative on an appropriate form (as described in 3 above). Where no relative can be traced the medical images may be used with the approval of the clinician in charge, but MUST be strictly anonymised.
All cases of images of deceased patients for whom relatives cannot
be found to provide ‘assent’/agreement should be referred to the
MIDRIB Medical Ethics Panel (see E below). Equally the Legal Liaison
Department of the Trust should be informed.
(D) Children
Particular care is required when using medical images of children
under the age of 16 and in the practical implications for them in
participating in their creation. Medical images may be made AFTER a parent
(or someone with legal parental responsibility) has given written consent
on an appropriate form, after having read the relevant information sheet.
Specific permission for the use by MIDRIB must be further obtained
on an appropriate form after the parent and child have read the associated
information sheet(s).
Specific permission for the use of medical images by MIDRIB must be obtained on an appropriate form after the parent has read the associated information sheet.
The need for photography should always be entered into the clinical record. The child’s views should always be sought and consent obtained when the child is competent to provide it. Written informed consent is usually necessary for photography, but there may be difficulty obtaining this from those with parental responsibility especially when they regard themselves as under suspicion. In certain situations (e.g. severe injury or injuries which may heal rapidly) images may be generated without the parent’s (or guardian’s) consent PROVIDING this is recorded in the patient’s notes in the usual way together with an independent consultant’s written view that the images are necessary and in the child’s best interests.
A child who is able to understand fully the nature and purpose of the images can provide written consent. A child may not be competent to do so, the parent may refuse or it may be inappropriate to ask the parent. In such circumstances, if there is doubt about the necessity of making the images, then the facts MUST be discussed with the responsible consultant and Social Services and legal advise may be required.
Medical images of children should ONLY be used for teaching, education and research AFTER obtaining written consent from the appropriate persons (a person with parental responsibility PLUS the child, if competent) and strict anonymity is preserved. Images for possible forensic or legal purposes should fulfil evidential requirements, but should respect confidentiality.
Where there is doubt on how to proceed, this should be discussed with the designated Child Protection Consultant and staff of the Department of Medical Illustration at an early stage.
Specific permission for the use of medical images by MIDRIB must be
further obtained on an appropriate form after the parent/guardian (and
child where appropriate) have read the associated information sheet(s).
(E) Historical Collections
Historical collections of medical images may be used without patients’ consent in certain circumstances, but patient’s consent should always be sought where it is practicable to do so.
It is difficult to define when medical images in historical collections may be used in every case. MIDRIB will set up a Medical Ethics Panel to consider individual cases on their merits. The factors that the Panel will consider might include:
~ The age of the images.
~ Whether the images were produced according to the ethical procedures that existed in the Trust at the time they were created.
~ Whether the patient’s identity is known, the patient might reasonably be traced and if they are likely to still be alive.
~ Whether the medical images are anonymous or can be made anonymous (for example, is the face, or other distinct features from which a person would be recognised, shown?).
~ The medical importance or uniqueness of the medical images.
~ Other factors appropriate to the individual circumstances.
The brief of the MIDRIB Medical Ethics Panel will be to ensure
optimal consistency with the policies stated throughout this document.
Users of medical images for educational and research purposes must
understand that a major factor in the approval of already existing
collections - aside from the strict anonymity which has been consistently
stressed - will be the effort which they make to determine the degree to
which the medical images have been created in conformity with MIDRIB
policy. For example, users should indicate what type of consent, if any,
was given for the original creation of the medical image; whether or not
they have made attempts to contact clinicians, patients and relatives
associated with the creation of each image; and the justification for
wishing to use these medical images rather than creating a new medical
image with patient consent which will achieve the same education or
research purpose.
Legal Advice
Publication of medical images of children in the context of child abuse, should NEVER occur unless specific legal advice has been obtained from the Legal Liaison Department of the Trust involved.
The Legal Liaison Department should also be consulted in any
case where there is doubt on the appropriate processes to follow.
Confidentiality and Copyright
All medical images remain the physical and copyright property of
copyright holder, normally the Trust in which they are made. MIDRIB will
not publish material without written permission from the copyright holder.
Produced for MIDRIB with advice from :
Len Doyal. Professor of Medical Ethics , University of
London
Annex A : The
following medical images DO NOT require patient permission where all
labels identifying the patient directly or indirectly have been
permanently removed :
i. Biopsies, Histological Samples, Tissue / Fluid sample
ii. Electrophoretic gel, for example :
~ Southern blot (DNA)
~ Western blot (protein)
iii. Electron micrograph, for example:
~ Scanning (SEM)
~ Transmission (TEM)
iv. Chemical/atomic structures
v. ECG
vi. EEG
vii. Evoked potential
viii. Graphs (epidemiology)
ix. Light micrographs, for example :
~ Chromosomal banding
~ Cytoscopy
~ Faecal smear
~ Karotype
~ Tissue sections
~ Tissue smears
~ Serology
~ Morphology (aetiological agent) - Whole mount
x. Gene sequence data
xi. Biochemistry report
xii. Blood report
xiii. Radiographs, for example:
~ Angiogram
~ Ultrasound
- Doppler
xiv. Micro-organisms, parasites
Patient consent is not required for medical images of discards.
In other cases, such as X-rays, patients consent should be sought where
this is reasonably practical. This list is not intended to be complete or
comprehensive. Other categories where there may be reasonable doubt about
whether patient consent is required before they are used in MIDRIB may be
considered an ethical panel.
Patient’s consent is required for clinical
photographs, and paintings and diagrams where the patient is identifiable.
Annex B : Items not to be included in MIDRIB at this stage
Medical images in the following categories of patients will not
be eligible for inclusion in MIDRIB at this stage:
Annex D — Image Description Forms

Annex E — Patient Consent Forms
MEDICAL IMAGES DIGITISED REFERENCE IMAGE BANK (‘MIDRIB’)
Photographs and other medical images are very useful to teach healthcare professionals and trainees, such as junior doctors, medical, radiography, physiotherapy and nursing students. For example, these can demonstrate what a particular illnesses looks like and help explain how to diagnose and treat them. These ‘medical images’ might include slides, photographs, films or video or voice recordings.
At present, medical images used for education and research are kept in a variety of places in collections of varying size and quality. The aim of the MIDRIB project is to collect and catalogue a comprehensive range of high quality medical illustrations and, through modern electronic technology, to ‘digitise’ them so they can be readily available to a wide range of authorised users.
Your doctor has contacted you on behalf of the MIDRIB project because they believe there may be medical images associated with your treatment or clinical situation, which could be useful for education and research purposes.
MIDRIB will not use any medical images of you without your explicit written consent. To help you make an informed decision about this, we have answered below some specific questions, which you may consider relevant.
What is a digitised image? What are their advantages?
Any static or moving image can be electronically converted into a series of numbers. These digitised images can then be used by computers to redisplay the image.
A digitised image can be reproduced many times without any loss in clarity. They can be passed between computers across networks and large numbers of digitised images can be stored on a single compact disk (sometimes called a CD-ROM).
Will you be recognisable on the images?
Most images used in medical education and research are anonymous in that it is impossible to identity the patient involved. Images of you will not be used by MIDRIB without your written consent. Before you give this consent, you should be given information about what type of image are involved, what they may be used for, and whether or not there is any chance that you may be recognised if they are used (or if in these circumstances they can be made completely anonymous). MIDRIB will not give your name to users of the service.
Who will see the images in MIDRIB?
Access to the medical images in MIDRIB will be restricted to appropriate healthcare professionals and trainees. If you consent to the use of an image from which you might be identified, this will normally only be available to registered medical libraries on CD-ROMs or with restricted access if distributed over computer networks.
Where will the images be seen?
The image collection, or parts of it, may be distributed on compact disks or be for use in network information systems with access restricted to universities and other academic users and researchers. The images may be accessed by staff and students of selected higher education and other institutions involved in the education and training of health professionals or in research associated with healthcare.
How will the images be used?
MIDRIB will distribute the medical images to authorised users for selected uses only, such as the production of teaching materials and for research. Occasionally images may be included in a commercial products for healthcare education, but only by respectable organisations of integrity. MIDRIB is non-profit making - any charges made to authorised users to be connected to MIDRIB or to receive compact disks of the images will go towards the running costs of providing the MIDRIB service.
How can you help?
We have tried to explain the type of images that we wish to included in MIDRIB and the uses to which they may be put - that is normally for educational or research purposes. If having considered this information, you are happy for us to use your image in the ways described above, we invite you to confirm this by signing the MIDRIB Consent Form.
If at some stage any of the users wish to use any of these images for publication, say in a textbook or healthcare journal, the author or your doctor should contact you to request your consent for this additional use.
Would you like more information?
If you need any further information, please talk to the person who gave you this information sheet. They will be happy to answer your questions.
Please remember that you do not have to give your consent. If you
decide not to give consent, it will not affect the quality of healthcare
you receive in any way.
Produced by:
MIDRIB, St. George’s Hospital Medical School,
Cranmer Terrace, London, SW17 ORE.
MIDRIB Patient Consent Form for the use of images.
The following are draft forms that might be used in connection with the collection and authorisation of medical images used in MIDRIB. In SGHMS we might use the same (or similar) form to that used by SGHMS at the moment (that is with the diagrams showing what medical images are to be created), but possibly:
* with the consent signature on the same side.
* adding the clinician’s signature.
* allowing the creation of images for digitisation by MIDRIB (but
NOT for education and training use without a consent form where this is
required).
The suggestion is that initially all images created at SGHMS
are passed to MIDRIB for possible inclusion in the service. The
photographer will ask the patients to sign the consent form and, where
consent is given, the 35mm slide is passed either:
a) To the clinician to approve and then on to MIDRIB to digitise. Then a copyright agreement is created and returned to the clinician to authorise and add descriptions for the images in batches. Only when this is returned will the images become ‘live’ on MIDRIB.
b) To MIDRIB to select, digitise and return the images to the
clinician to approve individual consent forms (or not) and to sign the
copyright agreement on behalf of the School / Trust and add descriptions.
Only when this is returned will the images become ‘live’ on
MIDRIB.
This will allow a large number of basic images to be collected.
After a short while, when sufficient basic images are collected for
MIDRIB, the photographer will only send images to MIDRIB when requested to
do so by the clinician. This reduces the administration costs, but should
allow all ‘interesting’ new images to be collected. The
photographer may continue to follow (a) in selected areas where standard
images are still required.
Patient Consent Form for the use of Medical Images in MIDRIB
I understand that the photography / video recording / ............................... (delete or add as appropriate) ‘medical images’ which, have been produced of me and form part of my confidential treatment record, may be useful for the purpose of medical and other healthcare related teaching, education and research.
In view of the explanations that I have received and the written information on the Medical Images Digitised Reference Image Bank (‘MIDRIB’) given to me, I also agree that these medical images may be included in MIDRIB and shown via this service to appropriate healthcare professional staff and students and subsequently used for the purpose of education and research. as permitted by MIDRIB.
I understand that if any medical image revealing my face or my identity is published in any other way, such as by reproduction in a medical journal, textbook or similar publications, then my consent for the use of these images will be specifically sought in each case.
Patient’s
Parent or Guardian’s
Relative’s Signature ............................................................ If patients decide not to give consent, it will not affect the quality of the healthcare they receive in any way.
~ ~ ~ ~ ~
I have show the patient the form explaining MIDRIB and
offered to answer questions.
Photographer’s Signature ............................................................ Date..................
~ ~ ~ ~ ~
I approve that the images attached may be used for the
purposes described above and confirm that appropriate informed consent has
been obtained according to the Code of Responsible Practice for the
inclusion of Medical Images in MIDRIB.
Clinician in Charge’s
Signature ............................................................
Date.........................
Note: Where the patient is under 16 the parent /
guardian must also sign. In exceptional circumstances (for example small
children or the patient is unconscious), a close relative may sign on the
patient’s behalf. This consent form should ONLY be approved by the
clinician (i) where these circumstances are covered by the Code of
Practice and (ii) the details of these circumstance s are given on this
form.
Annex F —
Guidelines for Contributors
Image / Set / Collection details
Information supplied by contributor Reference number Ref no on slide / image.
Is this image part of a set or collection? Part of a set / part of a collection - relationship with other images. Set name If image is part of a set.
Collection name If image is part of a collection.
Related image numbers Associated set numbers, collection numbers, if necessary. ------------------------------------------------------------------------------------------------------------------------
Date of original
Type of image submitted Image modality and type, e.g. still image / animation / text / 3D / courseware, etc. Title Descriptive single sentence.
Description
Contributor suggested keywords
------------------------------------------------------------------------------------------------------------------------
Access level e.g. undergraduate, junior doctor, reserved collection
Disease / condition name and description e.g. disease or syndrome name, synonyms, ‘popular’
(option to specify more than one) Names, visible symptoms, stage of disease, special
features to note. Organism Genus, species, common name, life cycle stage.
(option to specify more than one)
Geographical location City / town, country, geographical region.
-----------------------------------------------------------------------------------------------------------------------
Patient / image subject / population
Age e.g. prenatal, neonatal, months, years.
Sex Male / female / unknown.
View Dorsal / ventral / other
Setting e.g. clinic, field clinic, health centre, hospital, other
Relevant history
------------------------------------------------------------------------------------------------------------------------
Microscopy
Type of micrograph e.g. light, fluorescent, phase contrast, SEM, TEM,
other.
Magnification e.g. low (x 1-4), medium (x 10-20), high (x 40-100).
Preparation e.g. longitudinal section, transverse section, smear,
whole mount, thin blood film, thick blood film, other.
Histological stain e.g. Giemsa, van Gieson, Gram, H&E, Levaditi’s,
Papanicolaou, PAS, ZN, other (full name,
abbreviation).
Cells visible
Tissue
Organ
------------------------------------------------------------------------------------------------------------------------
Gross pathology
Specimen type Post mortem, surgical.
Organ
Preservation
Scale Centimetre (cm), millimetre (mm), other.
View Dorsal, ventral, longitudinal section, transverse
section, oblique section, other
Medical Imaging
Technique Angiogram, CAT scan, constrast x-ray, plain x-ray,
PET scan, SPECT scan, ultrasound scan, other.
View Dorsal, ventral, other.
Part of body/organ
------------------------------------------------------------------------------------------------------------------------
Other information Information which is not covered in the above sections. References Publications directly relating to this image.
Finished
Signed off
Guidelines for Contributors
Not all sections will be applicable to every image.
Select and complete all relevant sections. Please be as expansive as
possible, remembering that each image can only be retrieved by the textual
information that you supply here. While additional indexing terms will be
added by the MIDRIB indexer, these will be derived only from the text that
you make available. There is no limit to the amount of text you may
provide: feel free to attach securely additional pages if you feel
that insufficient space is provided here; if you choose to do so, please
label the additional pages unambiguously, so that the correct text is
finally associated with the correct image. If in doubt, err on the side of
caution and supply as much detail as you wish. Do not hesitate to repeat
or reinforce information.
Reference number (if applicable) - Indicate
your own reference number for this slide / image, as displayed on the
slide / image itself.
Sets and collections
Date of original - Supply the date that the
image was originally captured, to the best of your knowledge. If unsure,
give your own assessment of that date, but please also indicate your
uncertainty.
Type of image submitted - Please choose an
image type from the list supplied. Please be as comprehensive in your
description as possible.
Title - Provide a single, concise descriptive
sentence detailing the salient information about this image. This
sentence, if sufficiently descriptive, is likely to be used as the image
caption.
Description - Please give a detailed and accurate description of the image, stating (1) what the image actually shows, (2) any special features, (3) relevant background information, (4) why this image may be of interest to others in your specialty or in other specialties. Avoid sensitive terminology. Please do not assume that any features will be recognisable. Include details of any clinical feature, examination procedure etc. Importantly, please describe in detail the anatomical region and sub-region displayed. If there are other images, which relate to the same patient, or to the same set or collection, refer unambiguously to these, too.
Use the introductory few sentences to provide a brief
description. Use the following sentences to provide a more detailed
description and to provide relevant background information. Orientate the
user towards specific points if appropriate, using directions (right /
left) or position of the hand on the clock face (o’clock). Please
note that a full description of the image is essential, so that users can
easily retrieve this image from the database. In addition, comprehensive
descriptions enable other users to evaluate how useful an image may be for
their own purposes. Finally, a full description will enable the indexer to
assign a full range of appropriate indexing terms. For these reasons,
there is no limit on the length of the image description. Feel free to
attach securely additional pages if you feel that insufficient
space is provided here; if you choose to do so, please label the
additional pages unambiguously, so that the correct text is finally
associated with the correct image. If in doubt, err on the side of caution
and supply as much detail as you have available. Do not hesitate to repeat
or reinforce information.
Contributor suggested keywords - Underline in
the above description the terms that you believe are key in specifying the
subject of the image. Please suggest any additional keywords by which it
would be useful to be able to retrieve this image; as well as being
directly searchable, these will aid the indexer in assigning formal
indexing terms derived from MeSH, the Medical Subject Headings used to
index Medline and other related resources. If you customarily assign such
keywords yourself, please specify whether these terms derive from a
recognised standard vocabulary or thesaurus (e.g. ICD9, ICD10, SNOMED,
Read codes) and identify the source coding system explicitly.
Access level - Please indicate whether this
image would be of interest to a particular group of users e.g.
undergraduate, junior doctor, etc. Please also indicate whether this image
should be accessible widely or in a restricted fashion, e.g. retained in a
reserve collection.
Disease / condition name and description
- Give details of the primary disease / condition associated with the
image. If there is more than one disease associated with the image, enter
also the additional information, making sure to distinguish each one
clearly. For each disease, state :
Please give this information as specifically and in as
much detail as possible. The following are particularly important: visible
symptoms, stage of disease, special features to note, synonyms by which a
disease or condition may otherwise be known, especially ‘popular’
names of diseases and conditions (e.g. brittle bone disease).
Organism - Give details of the organism
associated with the image. If there is more than one organism associated
with the image, enter also the additional information, making sure to
distinguish each one clearly. Where known, please indicate the organism’s
genus, species, common name, life cycle stage. This section is
particularly applicable to images, which illustrate:
Geographical location - Wherever known, and if
it is important with regard to the image, or if a disease / condition or
organism is known to be location-specific, please indicate the city or
town, country and geographical region relevant to this image. In addition,
please give details of the location when the image shows any aspect of the
environment. State the country name and other known details when the image
relates to:
Patient / image subject / population - Give as much information as possible on the image subjects illustrated. Please be as specific as possible, e.g. for ages please indicate precise age if known. See separate notes for details of patient permission required.
Age e.g. prenatal, neonatal, months, years.
Sex male / female / unknown.
View dorsal / ventral / other.
Setting clinic, field clinic, health centre, hospital, other.
Relevant history
Image types
Type of micrograph, e.g. light, fluorescent, phase contrast, SEM, TEM, other.
Magnification e.g. low (x 1-4), medium (x 10-20), high (x 40-100).
Preparation e.g. longitudinal section, transverse section, smear, whole mount, thin blood film, thick blood film, other.
Histological stain e.g. Giemsa, van Gieson, Gram, H&E, Levaditi’s, Papanicolaou, PAS, ZN, other (full name, abbreviation)
Cells visible
Tissue
Organ
Specimen type Post mortem, surgical.
Organ
Preservation
Scale Centimetre (cm), millimetre (mm), other.
View Dorsal, ventral, longitudinal section,
transverse section, oblique section, other.
Technique Angiogram, CAT scan, constrast x-ray, plain x-ray, PET scan, SPECT scan, ultrasound scan, other
View Dorsal, ventral, other
Part of body/organ
Other information - Please provide any
further relevant information, which is not covered in the above sections.
References - Please give the full
references of any publications relating directly to this image. For
instance, please supply the full journal reference when the image refers
to a published paper, such as those describing specific epidemiological
surveys or new diagnostic techniques. Similarly, please provide full
references for books, book chapters, conference proceedings, or other
publications relating to this image. If possible, please use the Vancouver
System of referencing - which applies the Uniform requirements for
manuscripts submitted to biomedical journals, as agreed by the
International Committee of Medical Journal Editors (see
http://www.ama-assn.org/public/journals/jama/sc6336.htm).
Annex G — Process
Flow-Charts, etc